理解汽化过氧化氢(VHP)消毒的关键参数
温度、相对湿度和相对饱和度的关系
由于汽化过氧化氢不会留下残留物,并且在室温消毒非常有效,因此广泛用于隔离器、传递仓和不同设施的消毒。
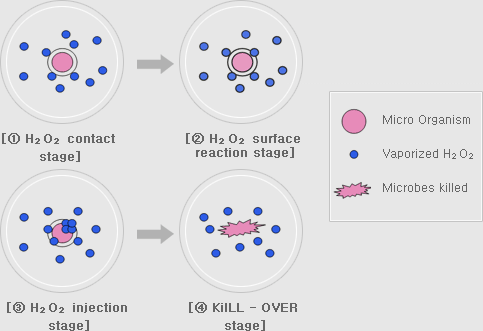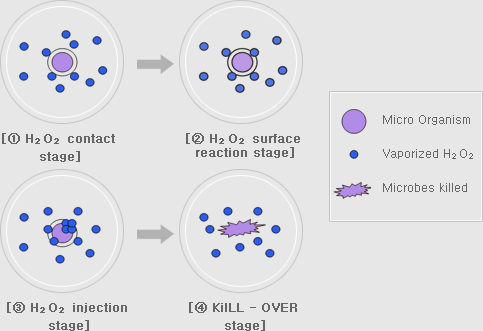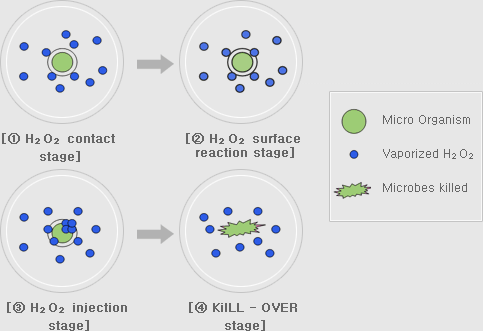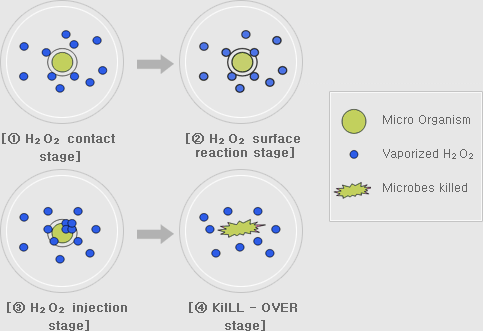
微生物可以在不同的湿度和 H2O2 ppm 水平下有效杀灭。一些生物净化舱或隔离器的制造商喜欢微冷凝技术;而另一些制造商则喜欢干雾技术,湿度保持远远低于凝露水平。但是,应避免使用喷雾冷凝,因对排气时间、材料和消毒效果均匀性可能造成负面影响。因此,在汽化过氧化氢(VHP)消毒过程中测量湿度至关重要。然而,水(H2O)和过氧化氢(H2O2)的分子结构非常相似。因此,它们都会影响空气的湿度。
相对湿度由其定义表示空气的湿度仅由水蒸气引起。
因此,用于汽化过氧化氢(VHP)的湿度传感器通常在正常湿度传感器上使用催化层。催化层催化过氧化氢,使湿度传感器只测量水蒸气。测得的相对湿度表示空气的湿度仅由水蒸气引起。测量蒸汽状态下H2O2 时,相对饱和度是指示过氧化氢和水蒸气共同组成的空气中的湿度参数。当相对饱和度达到 100 % RS 时,空气混合物开始冷凝。相对饱和度是指示空气与水蒸气和汽化过氧化氢的混合物何时开始冷凝的唯一参数。因此,在消毒过程中,必须测量相对饱和值。
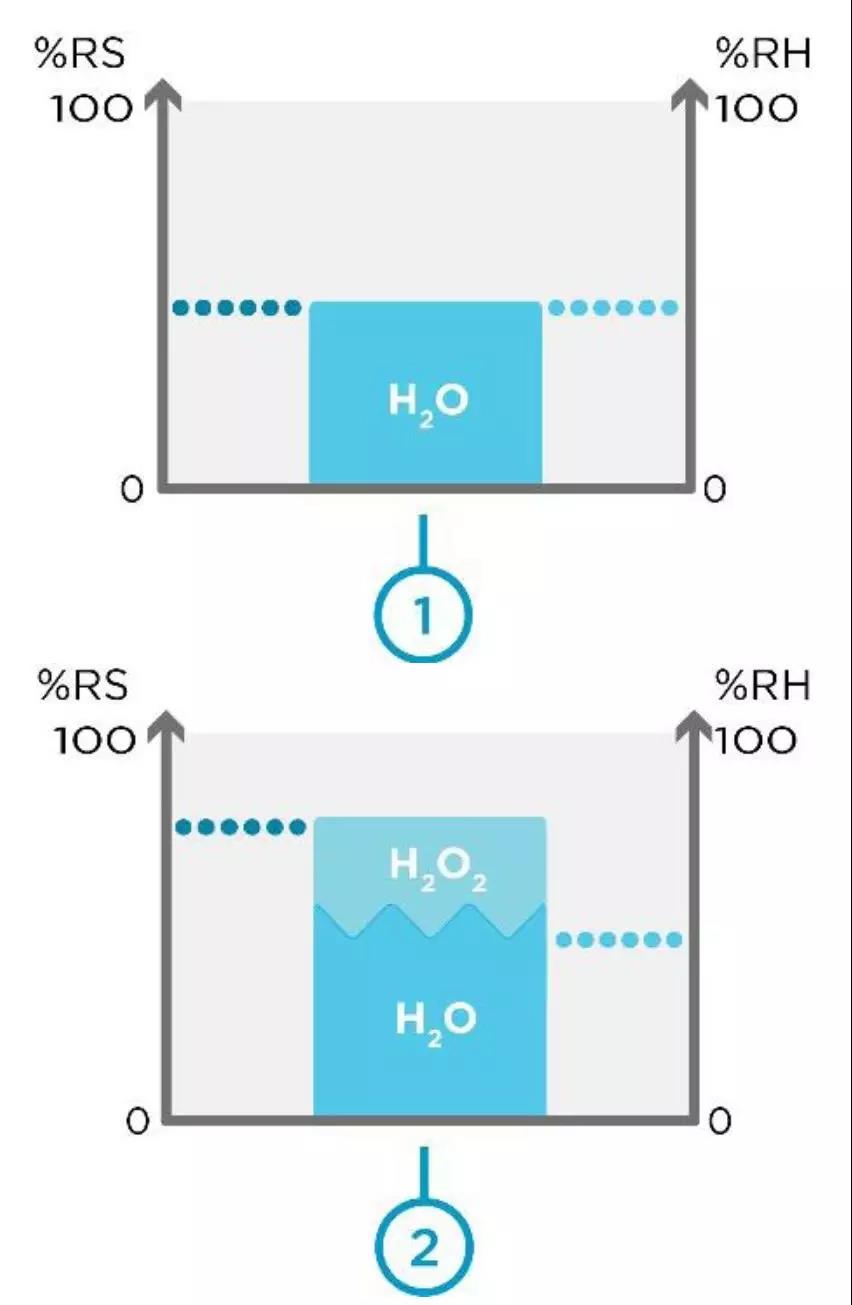
图 1.空间 1 不含 H2O2蒸汽,空间 2 包含 H2O2 蒸汽。
图1显示了两个不同的空间:空间1没有H2O2蒸汽,空间2有H2O2蒸汽。当不存在 H2O2 蒸汽时,相对饱和度等于相对湿度。这在空间1中可以看到。在空间2中,我们在相同体积的空气中注入了H2O2蒸汽。现在,相对饱和度高于相对湿度
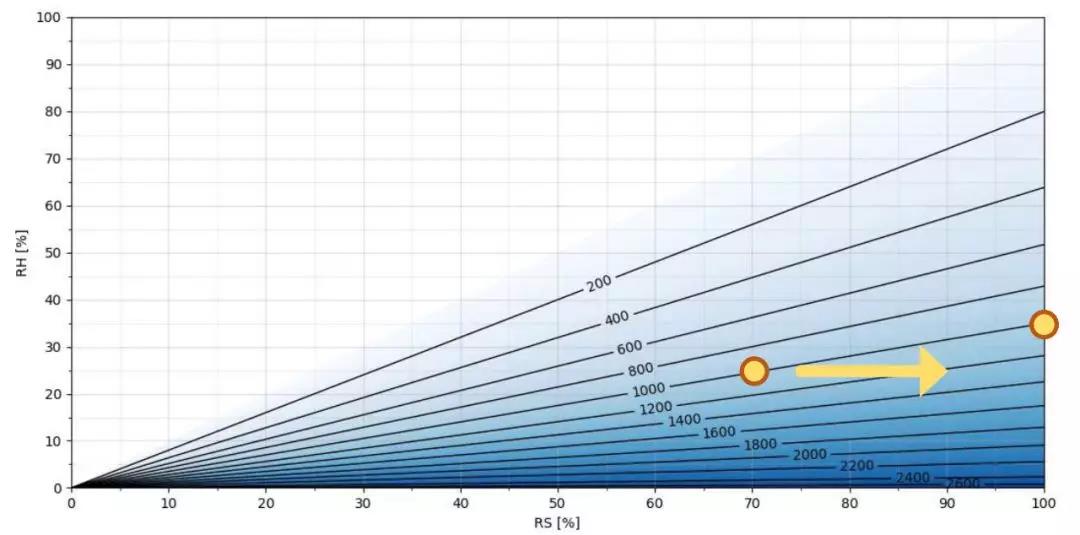
25℃下H2O2 ppm浓度与相对饱和度/相对湿度的关系
图 2 显示了不同浓度H2O2ppm 在 25℃下相对饱和度和相对湿度的函数。相对饱和度位于 x 轴上,相对湿度位于 y 轴上。较暗的阴影表示 H2O2 的 ppm 更高。正如你所看到的,空气混合物中的过氧化氢越多,相对饱和度和相对湿度值之间的差异就越大。例如,在 25℃下, 1000 ppm 过氧化氢,相对湿度 25%RH 与相对饱和度70%RS。当相对湿度为35%时,这种1000ppm气体混合物即开始冷凝。
温度影响冷凝前过氧化氢在空气中的含量(相对饱和度等于 100 %RS)。因此,当温度变化时,图也发生变化。
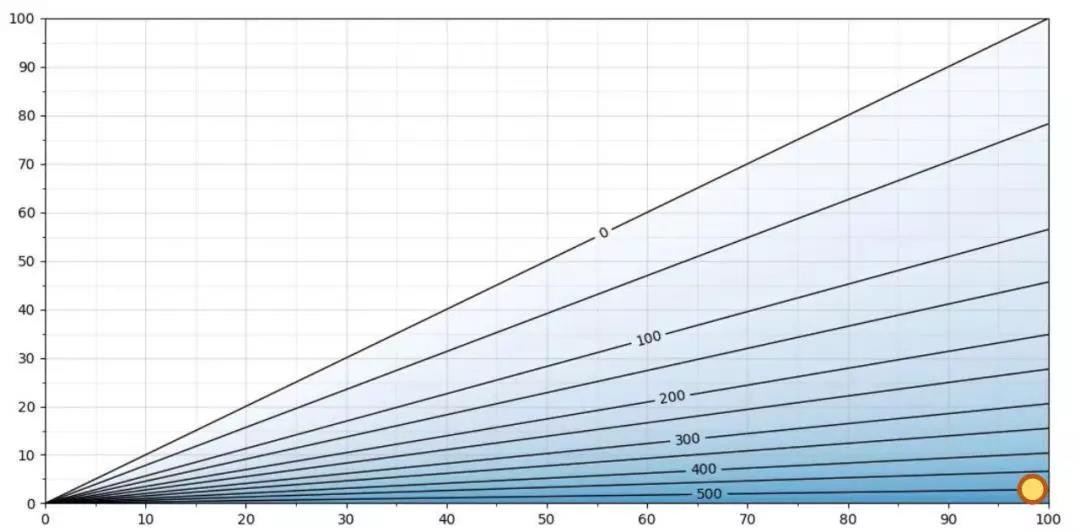
图3 5℃下H2O2浓度与相对饱和度/相对湿度的关系
图 3 显示了 5℃下的关系。5℃时的最大 H2O2 ppm 水平略高于 500 ppm。例如,在 5℃、500 ppm 过氧化氢和 100 %RS相对饱和度(微冷凝)条件下,相对湿度约为 2 %RH。由于相对饱和度为 100 %RS,空气混合物将冷凝。相对饱和度%RS 和相对湿度 %RH 在此温度下的差值十分明显:100 %RS vs. 2 %RH。在此情况下测量 %RH没有实际价值。
温度越高,冷凝前可注入到空气中 H2O2 ppm 越多;如图 4 和图 5 所示。在图 4 中,在 50℃条件下,H2O2浓度可以达到>12000 ppm。
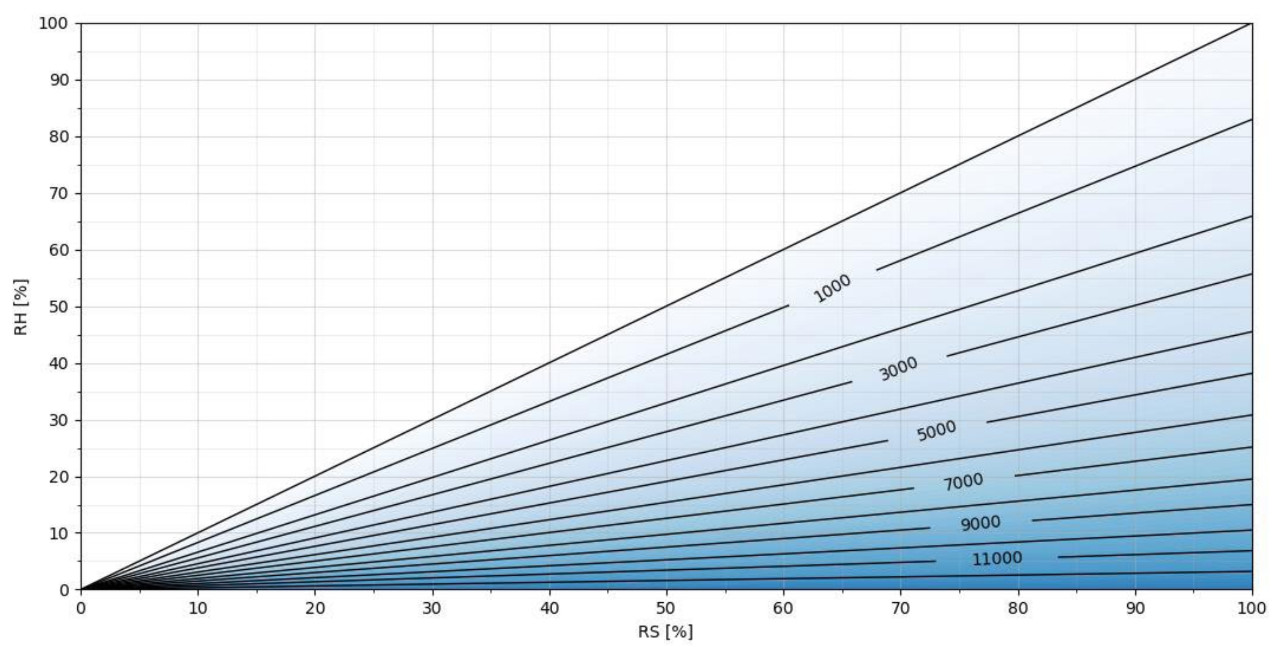
图4 50℃下H2O2浓度与相对饱和度/相对湿度的关系
图 5 表示不同相对湿度RH条件下,达到微冷凝(即相对饱和度=100%RS)所需的温度和H2O2浓度ppm关系。X轴为温度,y轴为H2O2 ppm 。曲线中的数值表示相对湿度。例如,在 20℃和 300 ppm 过氧化氢条件下,60%RH 相当于 100%RS(达到微冷凝)。如果将空气温度提高到 40℃,H2O2 浓度为 300 ppm,则相对湿度需要达到 87%RH,相对饱和度才为 100%RS。由于空气温度和H2O2浓度的关系,冷凝发生在<100%的相对湿度下。因此,温度越高,达到饱和的最大 RH%越高。如果我们在40℃时将过氧化氢水平从300ppm提高到900ppm,那么可达到的最大相对湿度将从87%RH降至70%RH。因此,ppm 浓度越高,最大 %RH 越低。
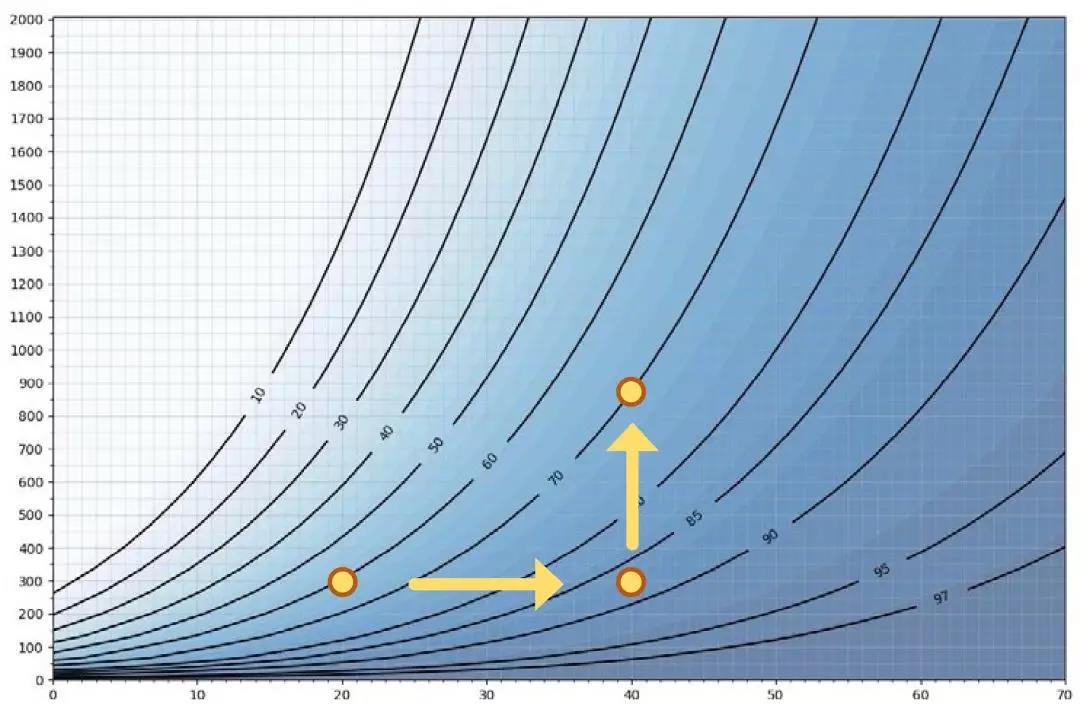
不同相对湿度RH条件下,达到微冷凝(即相对饱和度=100%RS)所需的温度和H2O2浓度ppm关系
这些数字说明了为什么在汽化过氧化氢(VHP)消毒过程中,仅仅关注相对湿度是不够的。注入 H2O2 的空气在±100% 的相对湿度下会冷凝,具体取决于空气的温度和过氧化氢的浓度。当空气混合物含有汽化H2O2时,相对湿度永远无法达到100%,因此几乎不可能准确估计冷凝何时发生。温度越高,需要达到的相对湿度越大。另一方面,H2O2浓度越高,可达到的最大相对湿度 RH 越低。
当用汽化过氧化氢(VHP)进行消毒时,相对饱和度是唯一能准确表示真实饱和度的参数,即,可以预期微冷凝的参数。